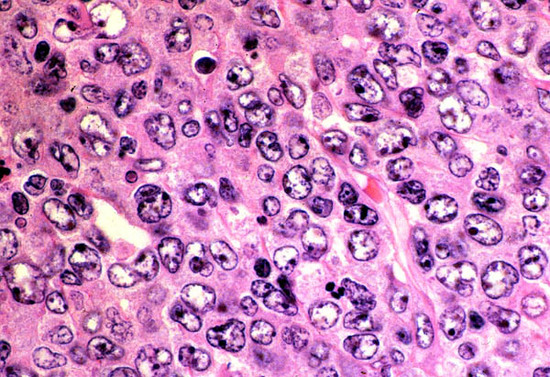
PIQUR Therapeutics AG, a Swiss clinical-stage pharmaceutical company, announced that the European Medicines Agency (EMA) has granted orphan drug designation to PIQUR’s lead compound PQR309 for the treatment of patients with diffuse large B-cell lymphoma (DLBCL).
“The EMA orphan drug designation for PQR309 in DLBCL is another important regulatory milestone, validating the potential therapeutic use of PQR309 in DLBCL,” said Claudia Pluess, Senior Regulatory Affairs Manager at PIQUR. Dr. Vladimir Cmiljanovic, chief executive officer of PIQUR, added, “PIQUR will continue to work with physicians and regulatory agencies to further define the clinical development strategy to bring a potential new treatment option to patients suffering from this disease.”
DLBCL is an aggressive form of lymphoma, and the most common type of non-Hodgkin lymphoma (NHL), accounting for about 30 percent of all NHL cases. The disease occurs primarily in older individuals, though it can also occur in children and young adults in rare cases. 10 to 15 percent of DLBCL patients exhibit refractory disease and an additional 20 to 25 percent relapse after initial response to therapy.
In addition to this orphan drug designation by the EMA in DLBCL, PIQUR has also recently received orphan drug designation from the FDA for PQR309 for the treatment of primary CNS lymphoma (PCNSL).
The EMA orphan drug designation is a status assigned to a medicine intended for use against a rare condition (prevalence of the condition in the European Union must not be more than 5 in 10,000) and allows a pharmaceutical company to benefit from incentives offered by the EU to develop a medicine for the treatment, prevention or diagnosis of a disease that is life threatening or a chronically debilitating rare disease.
Ema grants orphan drug designation, piqur’s, dlbcl