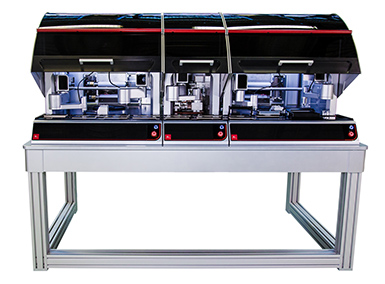
Chembio Diagnostics, a leader in point-of-care (POC) diagnostic tests for infectious diseases, has selected Ginolis Ltd, a global supplier of automation solutions, to deliver Ginolis’ Lateral Flow Device Assembly (LFDA) system to their manufacturing headquarters in Medford, New York.
The Ginolis Lateral Flow Device Assembly (LFDA) system is a compact solution that provides cost efficient assembly of rapid tests with high quality assurance. Based on Ginolis’ modular Xanthia automation platform, the LFDA is easily adaptable and can assemble different lateral flow devices with minimal product specific adjustments. Machine vision guidance and quality control throughout the entire system ensure a consistent high quality assembled device. The system will be used to automate assembly of Chembio’s patented Dual Path Platform (DPP) technology, which offers improved performance (sensitivity & specificity) over traditional lateral flow technology, as well as simultaneous detection capabilities of both antigen and antibody in a disposable rapid test format.
Ginolis VP of sales and marketing, Jorma Venäläinen, says “Ginolis sees intelligent automation as the future of diagnostics production. By being able to assemble multiple products with the same system, manufacturers save valuable space in their clean room environments. We are excited about working together with Chembio and delivering the first six module LFDA to USA.”
Ginolis to supply, lateral flow device assembly system, chembio diagnostics