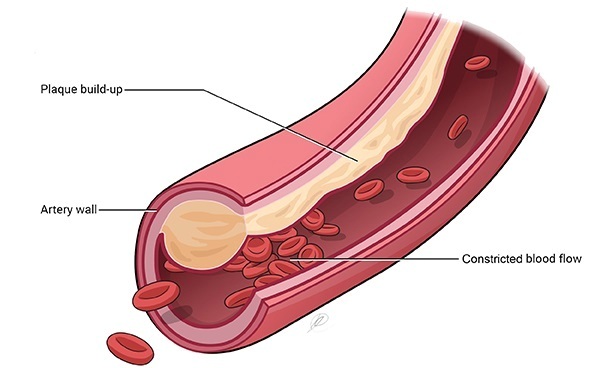
Regeneron Pharmaceuticals has announced that the US Food and Drug Administration (FDA) has granted Breakthrough Therapy Designation status to evinacumab for the treatment of hypercholesterolemia in patients with Homozygous Familial Hypercholesterolemia (HoFH), an inherited disorder that can lead to premature cardiovascular disease due to very high levels of LDL cholesterol. Evinacumab is an investigational monoclonal antibody to angiopoietin-like protein 3 (ANGPTL3). ANGPTL3 acts as an inhibitor of lipoprotein lipase and endothelial lipase, and appears to play a central role in lipoprotein metabolism.
Regeneron previously reported positive interim phase 2 results for evinacumab in HoFH patients and is currently planning a phase 3 trial. HoFH is the most severe form of hypercholesterolemia. While rare, occurring in approximately one to two people per million, untreated patients can have LDL cholesterol levels ranging from 500 to 1000 mg/dL, compared to normal LDL cholesterol levels of less than 130 mg/dL. Due to these high levels of LDL cholesterol, patients with HoFH are at an extreme risk of premature cardiovascular disease. Without treatment, patients typically present with signs and symptoms of atherosclerotic cardiovascular disease before the age of 20.
Breakthrough Therapy designation was created to expedite the development and review of drugs that target serious or lifethreatening conditions. A Breakthrough Therapy drug must show preliminary clinical evidence of a substantial improvement on a clinically significant endpoint over available therapies, or over placebo if there is no available therapy.
Regeneron is a leading science-based biopharmaceutical company that discovers, invents, develops, manufactures and commercializes medicines for the treatment of serious medical conditions. Regeneron commercializes medicines for eye diseases, high LDL-cholesterol, atopic dermatitis and a rare inflammatory condition and has product candidates in development in other areas of high unmet medical need, including rheumatoid arthritis, asthma, pain, cancer and infectious diseases.
Regeneron’s evinacumab, us fda breakthrough, treat homozygous, hypercholesterolemia