Zydus Cadila has received the final approval from the US FDA to market Tiadylt ER (diltiazem hydrochloride extended-release, USP) capsules, in strengths of 120 mg, 180 mg, 240 mg, 300 mg, 360 mg, and 420 mg.
Tiadylt ER capsules are used to treat hypertension (high blood pressure), angina (chest pain), and certain heart rhythm disorders. The drug will be manufactured at the group’s formulations manufacturing facility at the pharma SEZ, Ahmedabad.
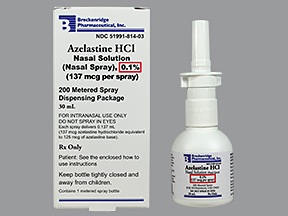
The company also received the final approval from the US FDA to market azelastine hydrochloride nasal spray, 137 mcg. Azelastine hydrochloride is used to relieve nasal symptoms such as runny/itching/stuffy nose, sneezing, and post-nasal drip caused by allergies or other conditions. It will be manufactured at the group’s formulations manufacturing facility at Moraiya, Ahmedabad.
The group now has more than 140 approvals and has so far filed over 300 ANDAs since the commencement of the filing process in FY 2003-04.
Zydus receives, us fda, tiadylt er capsules, azelastine hcl nasal spray