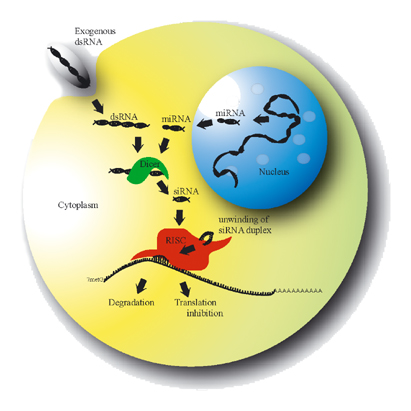
Sirnaomics, a leading biopharmaceutical company in discovery and development of RNAi therapeutics, has announced that the company had initiated a clinical phase IIa study for its leading siRNA therapeutic candidate, STP705 (Cotsiranib), for evaluation of the safety and efficacy of STP705 in human subjects with hypertrophic scar.
Sirnaomics lead product candidate, STP705, is an anti-fibrosis siRNA (small interfering RNA) therapeutic taking advantage of a dual-targeted inhibitory property and polypeptide nanoparticle (PNP)-enhanced delivery to directly diminish both fibrotic activity and inflammatory activity.
These attributes will allow application in many disease states. This initial clinical study is designed to measure the safety, tolerability, and efficacy for treatment of Skin Hypertrophic Scars caused as a result of abnormal healing following surgical procedures. Currently, there are no pharmaceutical products for hypertrophic scarring approved by US Food and Drug Administration or European Medicines Agency.
"This first-in-man study of STP705 in USA will provide safety and efficacy evidence of Sirnaomics’ unique siRNA drug design and the PNP formulation for a novel antifibrotic therapeutic approach”, said the founder and chief executive office of the company, Dr. Patrick Y. Lu, “The knowledge and experience from the intradermal injection of this siRNA therapeutic should pave a path towards utilizing this drug candidate systemically for other fibrotic disease treatments, addressing broader unmet clinical needs.”
“We are very excited to enter the clinic with STP705 in our first indication of hypertrophic scar. This IND approval serves as validation for our robust preclinical programs. We expect this rigorous clinical study design will enable us to gain great insights into the impact of STP705 on both fibrosis and inflammation and this information will enable us to greatly expand our platform and product pipeline for future disease indications”. The company’s chief medical officer, Michael Molyneaux, managing director and MBA, further emphasized.
Sirnaomics initiates, phase iia, study of sirna therapeutic, candidate, stp705, treat hypertrophic scar